Corvus Pill Promises Breakthrough in Early-Stage Eczema Trial
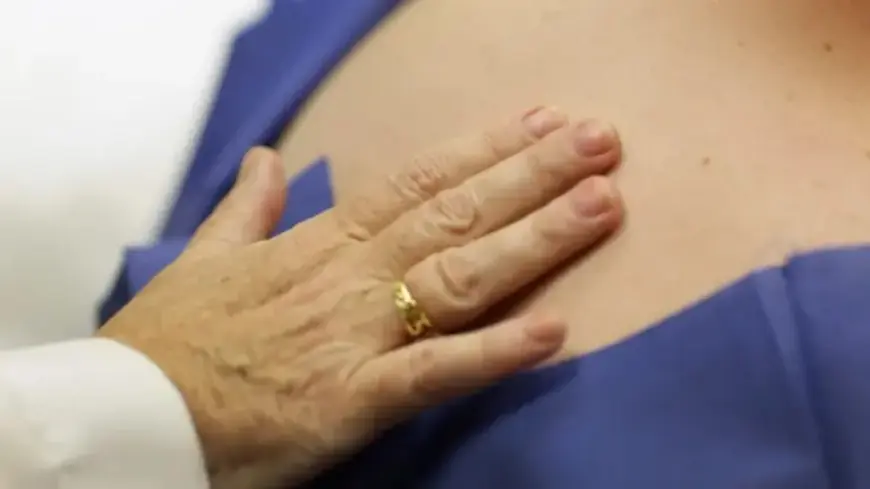
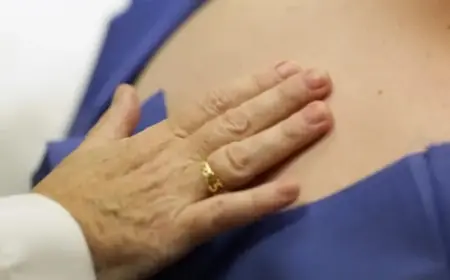
Corvus Pharmaceuticals has unveiled promising results from a recent early-stage trial of its new oral eczema treatment, soquelitinib. This medication could potentially surpass currently available treatments, notably Dupixent, a widely recognized therapy in the market.
Promising Trial Results
The trial outcomes were made public on Tuesday, revealing significant efficacy of soquelitinib. After a duration of eight weeks, a remarkable 75% of the participants who received the Corvus pill exhibited a minimum of 75% improvement in their skin condition. This measurement is commonly referred to as EASI 75, which assesses the extent and severity of skin lesions.
Comparison with Placebo
In contrast, only 20% of participants in the placebo group reached the same level of improvement. Furthermore, the study highlighted that 33% of those treated with soquelitinib achieved clear or nearly clear skin after eight weeks, according to physician assessments. Notably, none of the individuals in the placebo group achieved these results.
Potential Impact on Eczema Treatment
The implications of these findings could be significant for patients suffering from eczema. If soquelitinib continues to demonstrate effectiveness in subsequent trials, it may provide a new, more effective option for individuals seeking relief from this often debilitating condition. This progress in drug development could position Corvus Pharmaceuticals at the forefront of innovative eczema therapies.
Key Takeaways
- Drug Name: Soquelitinib
- Trial Duration: 8 weeks
- EASI 75 Improvement: 75% of treated participants
- Placebo Improvement: 20% of participants
- Clear Skin Achievement: 33% of treated participants
- Placebo Group Achievements: 0%
As the study progresses, further evaluations will be necessary to fully understand the long-term benefits and potential side effects of soquelitinib. Stay informed on advancements in the biotech industry and new treatments by following updates on Filmogaz.com.