Health Canada Alerts Public to Counterfeit GLP-1 Drug Risks
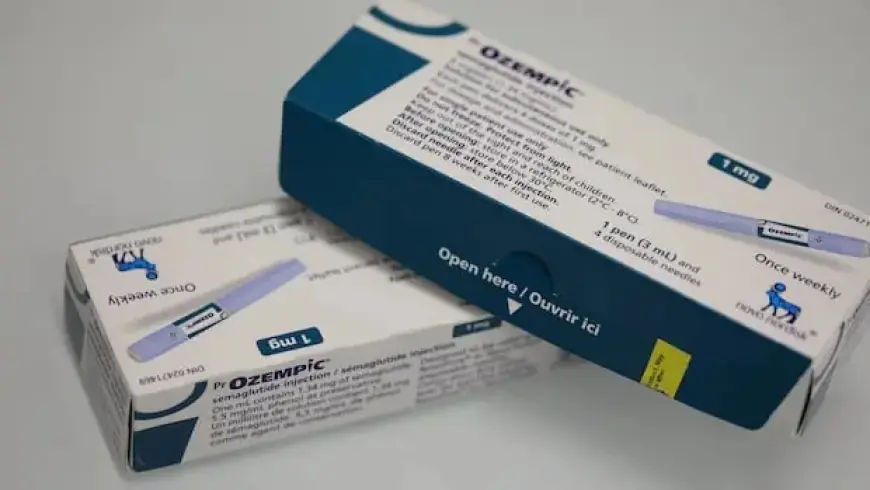
Health Canada has issued a warning to the public regarding the dangers of counterfeit GLP-1 drugs. These drugs, which include semaglutide, are popular for weight loss and are sold under brand names like Ozempic and Wegovy. The advisory emphasizes that unauthorized versions pose significant health risks due to their lack of assessment for safety and effectiveness.
Understanding GLP-1 Medications
The authorized GLP-1 medications in Canada include:
- Semaglutide (Ozempic and Wegovy injections, Rybelsus oral tablets)
- Tirzepatide (Mounjaro and Zepbound injections)
Health Canada has identified several retailers selling fake versions of these medications both in physical stores and online. Additionally, misleading online advertisements use fraudulent endorsements and logos mimicking official Health Canada branding to deceive consumers.
The Risks of Counterfeit Medications
Counterfeit or unauthorized GLP-1 products can lead to multiple health dangers, including:
- Incorrect dosages of active ingredients
- The presence of unlisted or harmful components
- Contaminants like heavy metals, solvents, or harmful microorganisms
- Improper labeling
- Unsafe manufacturing processes
Injectable counterfeit products are especially risky. They can cause infections, allergic reactions, and other severe health issues due to improper handling and contamination.
Legal Implications
In Canada, selling unauthorized drugs and making false health claims is illegal. Health Canada emphasizes that it does not endorse any health products and does not permit its logos to be used for marketing purposes.
Health Canada’s Recommendations
To ensure safety, Health Canada recommends the following:
- Purchase prescription medications only from licensed pharmacies.
- Avoid unauthorized or counterfeit products.
- Check for an eight-digit Drug Identification Number (DIN) on medication labels.
- Consult with healthcare professionals if you have used unauthorized GLP-1 products.
- Be aware of the risks associated with online drug purchases and select safe online pharmacies.
- Report any side effects or suspicious health products to Health Canada.
The regulatory body continues to monitor the market and collaborates with the Canada Border Services Agency to prevent unauthorized shipments from entering the country.
Recent warnings from the World Health Organization in June 2024 highlighted the detection of counterfeit semaglutide in countries including Brazil, the United Kingdom, and the United States. Consumers are urged to remain vigilant about the authenticity of medications they purchase.