FDA Challenges Misleading Claims in Novo’s Obesity Pill TV Ad
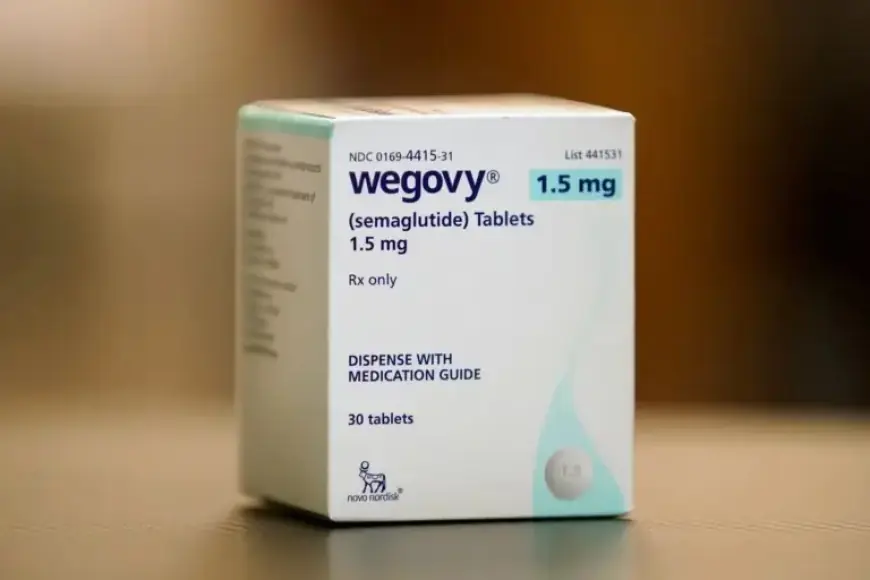
The U.S. Food and Drug Administration (FDA) has flagged issues with a television advertisement for Novo Nordisk’s weight-loss medication, Wegovy. The agency found that the ad contained “false or misleading” claims regarding the drug’s effectiveness in aiding weight loss. This revelation adds to the complications facing the pharmaceutical company.
FDA’s Concerns About Misleading Claims
The FDA criticized the advertisement for presenting Wegovy in pill form as superior to other weight-loss treatments. In a letter sent to Novo Nordisk on February 5, the FDA highlighted specific phrases from the commercial. The phrases “live lighter” and “a way forward” were noted as particularly misleading.
Key Issues Raised by the FDA
- The FDA asserts that the ad misrepresents Wegovy’s advantages.
- Specific phrases in the advertisement have been deemed deceptive.
- This scrutiny is part of the FDA’s broader commitment to overseeing drug marketing practices.
Novo Nordisk now faces the challenge of addressing these concerns while maintaining consumer trust. The company’s marketing strategies will likely undergo revisions to ensure compliance with FDA regulations. This incident serves as a reminder of the stringent standards governing pharmaceutical advertising.